
NanoBat
GHz nanoscale electrical and dielectric measurements
of the solid-electrolyte interphase
and applications in the battery manufacturing line
of the solid-electrolyte interphase
and applications in the battery manufacturing line
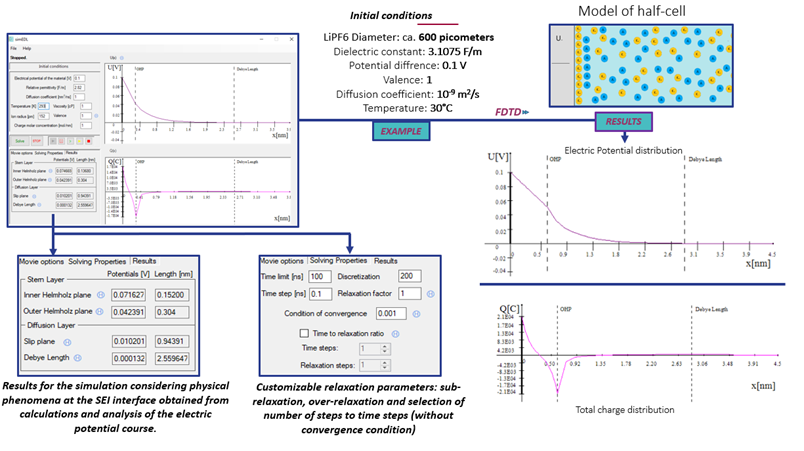
ELECTRIC DOUBLE LAYER SOLVER
Performance of batteries is strongly influenced by the formation of an interfacial layer between the electrode and electrolyte, called solid electrolyte interface (SEI). A complete description of the physical-chemical phenomena occurring at SEI is very complex and its rigorous modelling would require an enormous amount of computing power. For the sake of simplification, various approaches are used, known more generally as the electric double layer (EDL). In this case, we use a popular solution: the Stern model. It takes into account the adsorption of ions on the electrode surface, which causes the counter-ions to attract.
A half-cell system is considered in which the physical parameters of the electrolyte are known and the electric potential at the electrode is defined. It should be assumed that the Stern layer immediately appears next to the electrode, which is flat, uniformly charged and the ions adsorbed to it do not repel each other. In the diffusion part, the ions are treated as points and satisfy the Maxwell-Boltzmann equations. There is a continuous distribution of the dielectric constant εr and temperature T along the entire system. Values such as electron mobility µ and diffusion coefficients Dc are discretized in relation to a given space. The electric potential U changes according to the molar concentration n0 change due to the movement of the ions. This directly affects to the charge density ρ.
A half-cell system is considered in which the physical parameters of the electrolyte are known and the electric potential at the electrode is defined. It should be assumed that the Stern layer immediately appears next to the electrode, which is flat, uniformly charged and the ions adsorbed to it do not repel each other. In the diffusion part, the ions are treated as points and satisfy the Maxwell-Boltzmann equations. There is a continuous distribution of the dielectric constant εr and temperature T along the entire system. Values such as electron mobility µ and diffusion coefficients Dc are discretized in relation to a given space. The electric potential U changes according to the molar concentration n0 change due to the movement of the ions. This directly affects to the charge density ρ.

The NanoBat project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 861962.
